Summary of the Article
1. How does CO2 affect pH levels?
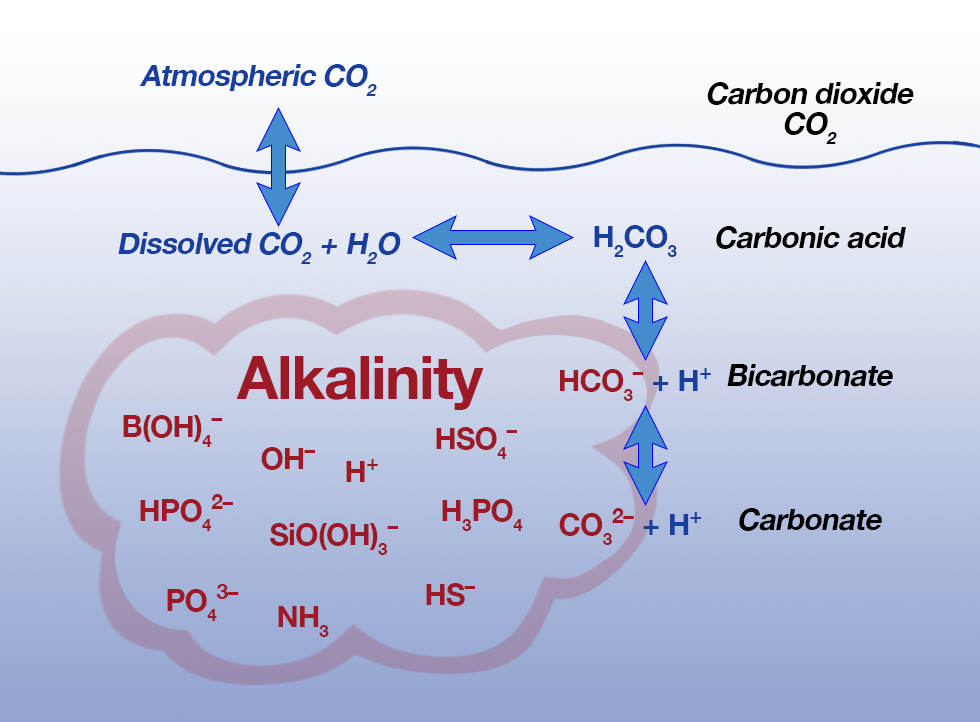
Carbon dioxide influences the pH of blood by reacting with water to form carbonic acid (H2CO3), which can dissociate to form a hydrogen ion (H+) and a hydrogen carbonate ion (HCO3-). Increasing the concentration of carbon dioxide in the blood therefore results in more H+ ions and a lower pH.
2. Does adding CO2 to water change pH?
Carbon dioxide can dissolve in water and then reacts with water to form carbonic acid. Since the acid then dissociates into carbonate ions and hydrogen ions and eventually forms H30+ ions, it follows that an increase in CO2 will cause a decrease in pH because the solution is getting more acidic.
3. Is CO2 alkaline?
Carbon dioxide is particularly influential in regulating pH. It is acidic, and its concentration is in continual flux as a result of its utilization by aquatic plants in photosynthesis and release in respiration of aquatic organisms.
4. What happens when you add CO2 to water?
When carbon dioxide reacts with water, carbonic acid is formed, from which hydrogen ions dissociate, increasing the acidity of the system.
5. How does CO2 affect alkalinity in water?
It should be noted that carbon dioxide does not decrease the alkalinity. It adds more carbonic acid to the system, which in turn lowers the pH because of the shifting of carbonate species ratios. The addition of carbon dioxide increases the overall carbonate in the system.
6. What is the relationship between pH, alkalinity, and CO2?
Higher carbon dioxide concentration will decrease pH slightly at a given alkalinity in freshwater, but it also will cause a higher alkalinity. As a result, the pH will rise slightly as the alkalinity increases.
7. Does CO2 raise alkalinity in water?
Higher carbon dioxide concentration will decrease pH slightly at a given alkalinity in freshwater, but it also will cause a higher alkalinity. As a result, the pH will rise slightly as the alkalinity increases.
8. How much will CO2 lower pH?
If we then add CO2 to 28ppm your pH would drop to 6.2, a change of 0.8. One possible explanation for this myth is that many copies of this pH chart skip some of the higher pH values, for example, jumping from pH 7.4 to a pH of 8.0.
How does CO2 affect pH levels
Carbon dioxide influences the pH of blood by reacting with water to form carbonic acid (H2CO3), which can dissociate to form a hydrogen ion (H+) and a hydrogen carbonate ion (HCO3-). Increasing the concentration of carbon dioxide in the blood therefore results in more H+ ions and a lower pH.
Does adding CO2 to water change pH
Carbon dioxide can dissolve in water and then reacts with water to form carbonic acid. Since the acid then dissociates into carbonate ions and hydrogen ions and eventually forms H30+ ions, it follows that an increase in CO2 will cause a decrease in pH because the solution is getting more acidic.
Cached
Is CO2 alkaline
Carbon dioxide is particularly influential in regulating pH. It is acidic, and its concentration is in continual flux as a result of its utilization by aquatic plants in photosynthesis and release in respiration of aquatic organisms.
Cached
What happens when you add CO2 to water
When carbon dioxide reacts with water, carbonic acid is formed, from which hydrogen ions dissociate, increasing the acidity of the system.
How does CO2 affect alkalinity in water
It should be noted that carbon dioxide does not decrease the alkalinity. It adds more carbonic acid to the system, which in turn low- ers the pH because of the shifting of carbonate species ratios. The addition of carbon dioxide increases the overall carbonate in the system.
What is the relationship between pH alkalinity and CO2
Higher carbon dioxide concentration will decrease pH slightly at a given alkalinity in freshwater, but it also will cause a higher alkalinity (Fig. 2). As a result, the pH will rise slightly as the alkalinity increases (Fig. 2).
Does CO2 raise alkalinity in water
Higher carbon dioxide concentration will decrease pH slightly at a given alkalinity in freshwater, but it also will cause a higher alkalinity (Fig. 2). As a result, the pH will rise slightly as the alkalinity increases (Fig. 2).
How much will CO2 lower pH
If we then add CO2 to 28ppm your pH would drop to 6.2, a change of 0.8. One possible explanation for this myth is that many copies of this pH chart skip some of the higher pH values, for example, jumping from pH 7.4 to a pH of 8.0.
Is CO2 acidic or alkalotic
Carbon dioxide, which is mildly acidic, is a waste product of the processing (metabolism) of oxygen and nutrients (which all cells need) and, as such, is constantly produced by cells.
What causes alkalinity to increase in water
If the landscape is in an area containing rocks such as limestone then the runoff picks up chemicals such as calcium carbonate (CaCO3), which raises the pH and alkalinity of the water. In areas where the geology contains large amounts of granite, for instance, lakes will have a lower alkalinity.
How much does CO2 lower pH
If we then add CO2 to 28ppm your pH would drop to 6.2, a change of 0.8. One possible explanation for this myth is that many copies of this pH chart skip some of the higher pH values, for example, jumping from pH 7.4 to a pH of 8.0.
What is the relationship between alkalinity and carbonate
The higher the alkalinity is, the higher the buffering capacity against pH changes. Alkalinity essentially becomes a measure of the buffering capacity of the Carbonate/Bicarbonate ions — and to some extent—the hydroxide ions of water.
Are alkalinity and pH correlated
As water alkalinity increases, there is a greater resistance to a change in water pH or, more importantly, a greater ability of the water to change the pH of something else like soils or potting mixes.
Does CO2 raise or lower pH in pool
Raise pH. Another thing you'll learn during pool service training is just as increased carbon dioxide (CO2) decreases pH, increased pH levels reduce carbon dioxide (CO2). This is accomplished by using additives that have a high pH to the water like sodium carbonate, non-stabilized chlorine, or sodium bicarbonate.
What increases alkalinity in water
If the landscape is in an area containing rocks such as limestone then the runoff picks up chemicals such as calcium carbonate (CaCO3), which raises the pH and alkalinity of the water.
Does CO2 booster change pH
Carbon dioxide impacts pH because it rapidly turns into carbonic acid when it enters the water. Acids lower pH, so more carbon dioxide means more carbonic acid, which means lower pH1. A calcium reactor can also lower a tank's pH since C02 is injected into the reactor.
What are the 3 causes of alkalinity of water
The alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks which contain carbonate, bicarbonate, and hydroxide compounds. Borates, silicates, and phosphates may also contribute to alkalinity.
What brings alkalinity up
Baking soda, also known as sodium bicarbonate is naturally alkaline, with a pH of 8. When you add baking soda to your pool water, you will raise both the pH and the alkalinity, improving stability and clarity. Many commercial pool products for raising alkalinity utilize baking soda as their main active ingredient.
Will CO2 booster lower pH
Carbon dioxide has an effect on the pH levels of aquariums, and this is important to know for all aquarists, particularly those who care for aquatic plants. The more CO2 generated or injected into aquarium water, the lower its pH level will be.
Does carbonate cause alkalinity
It is an aggregate measure of the sum of all titratable bases in the sample. Alkalinity in most natural waters is due to the presence of carbonate (CO3=), bicarbonate (HCO3-), and hydroxyl (OH-) anions. However, borates, phosphates, silicates, and other bases also contribute to alkalinity if present.
What does alkalinity depend on
Alkalinity is not a chemical in water, but, rather, it is a property of water that is dependent on the presence of certain chemicals in the water, such as bicarbonates, carbonates, and hydroxides.
What is the relationship between pH alkalinity and co2
Higher carbon dioxide concentration will decrease pH slightly at a given alkalinity in freshwater, but it also will cause a higher alkalinity (Fig. 2). As a result, the pH will rise slightly as the alkalinity increases (Fig. 2).
What causes high alkalinity
As it turns out, alkalinity rises due to excess hydroxides left behind by hypochlorite chlorines: sodium hypochlorite (liquid chlorine) and calcium hypochlorite (cal hypo). There is a minor net rise in TA when all things are fully oxidized in the water.
Does too much CO2 lower pH
As mentioned, when carbon dioxide is dissolved in water, the release of hydrogen ions in carbonic acid is what lowers the pH. As CO2 levels increase around Earth, the amount of dissolved CO2 also increases, which increases the amount of carbonic acid, therefore decreasing the pH.
What chemicals increase alkalinity
sodium bicarbonate
Common chemicals used to increase alkalinity and pH include: Calcium oxide or calcium hydroxide (as lime slurry) Sodium hydroxide (caustic soda) Sodium carbonate (soda ash) or sodium bicarbonate.